CHARACTERIZATION OF IMAGE DIFFERENTIATION
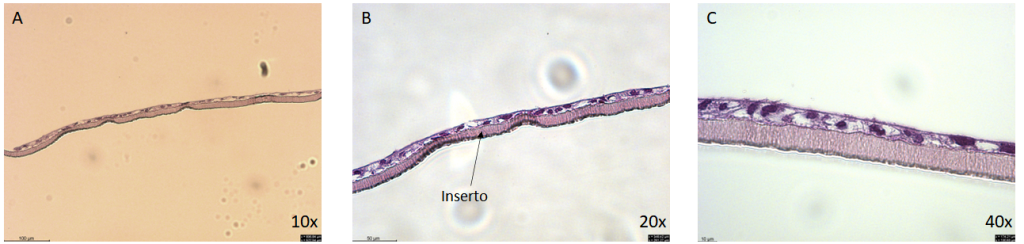
Intestinal barrier smouted with Hematoxylin & Eosin (H&E) after cuts of 5 to paraffin.
(A) Increase of 10x (B) 20x (C) 40x.
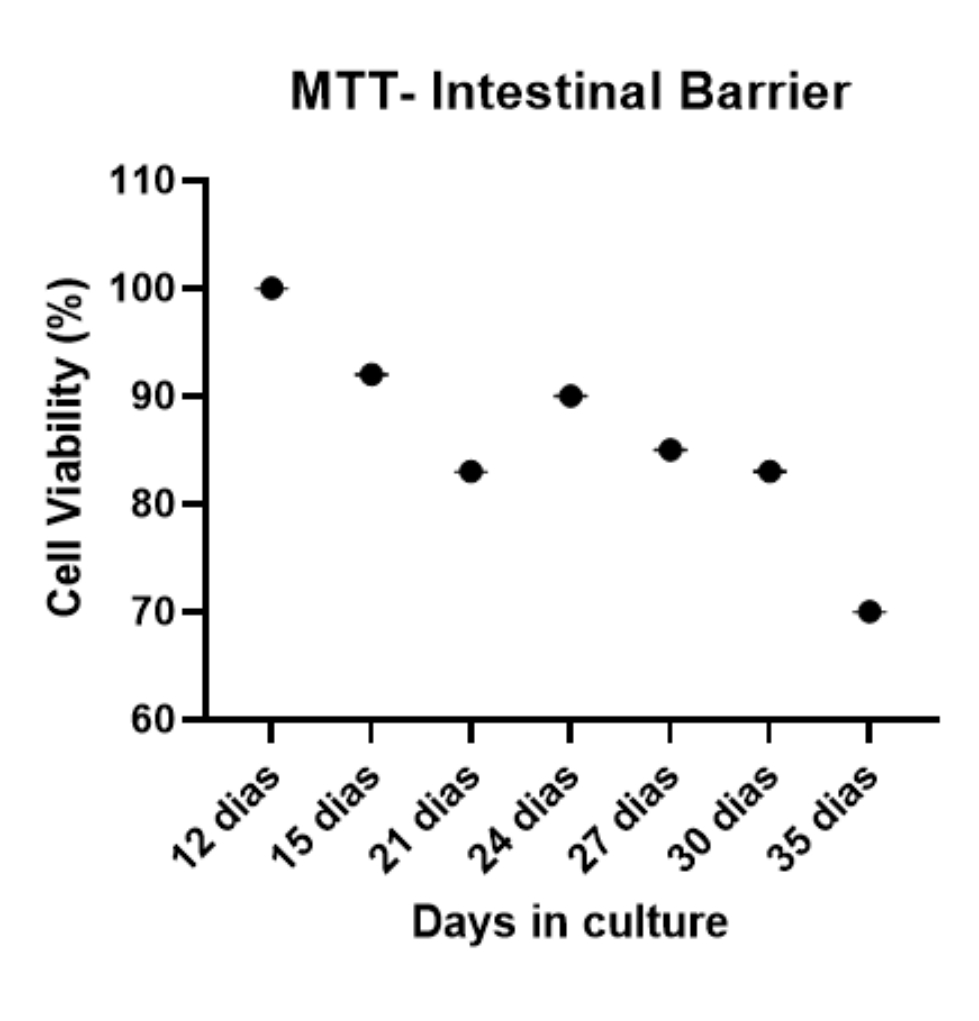
CELLULAR VIABILITY
Measure of cell viability (%) of intestinal barriers over time, showing the high viability of barriers (greater than 80%) for up to 35 days after their biofabrication.
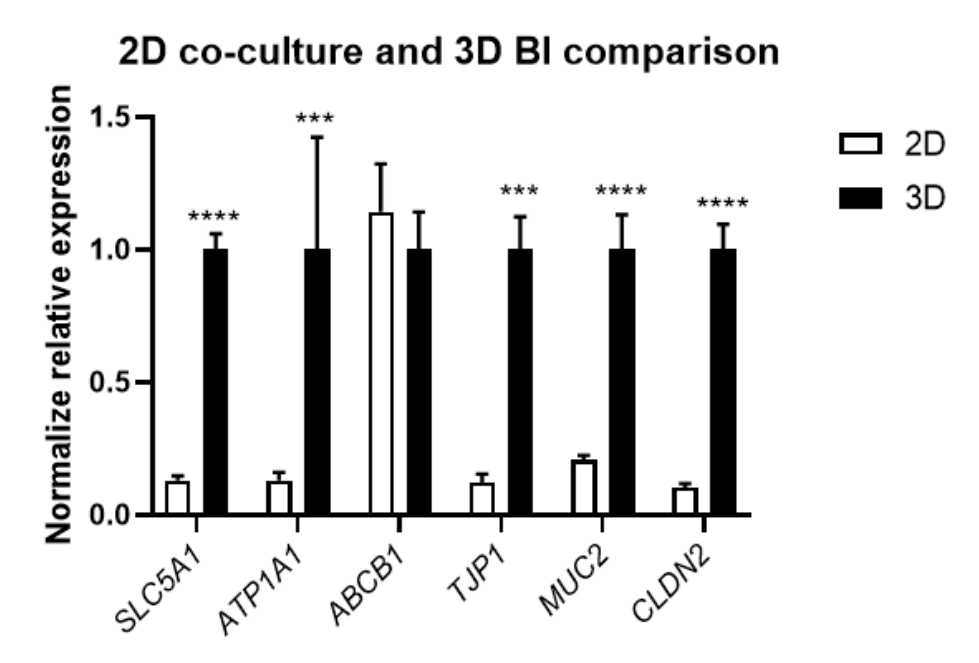
GENE EXPRESSION
Expression of markers of intestinal barrier function demonstrating the increase in expression in cell culture of B.I.3D, in relation to traditional 2D cultivation.
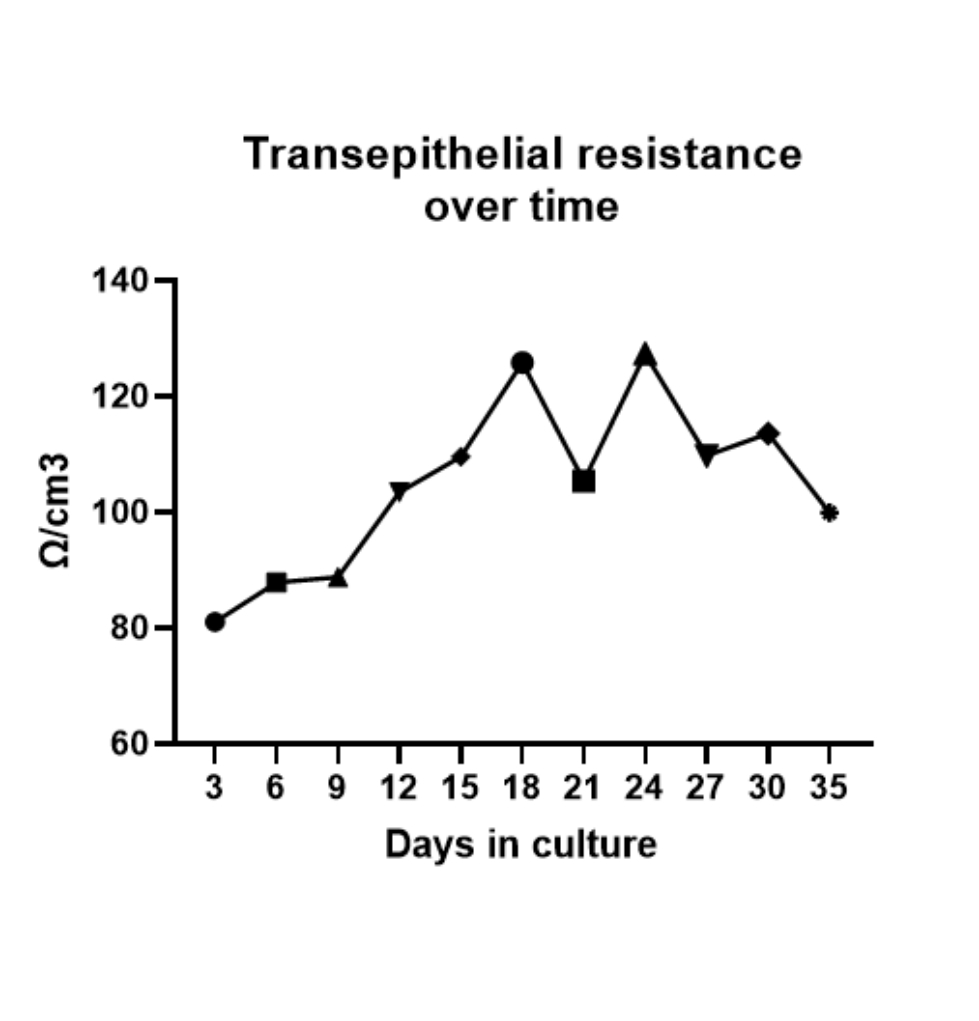
TRANSEPITHELIAL RESISTANCE
Measurement of transepithelial resistance during the differentiation of B.I. demonstrating that it has resistance as expected for a tissue.